
As technology continues to advance, laser products and instruments are playing an increasingly important role in people’s lives and work. In the U.S. market, FDA certification has become a necessary entry certificate for laser products and instruments. So, what is the standard and scope of FDA certification of laser products and instruments?
Ⅰ, The laser products and instruments FDA certification standards
FDA is the U.S. Food and Drug Administration (Food and Drug Administration), is responsible for regulating the U.S. market and its products. All laser products and instruments must be certified by the FDA in order to be sold and used in the U.S. market. FDA certification standards for laser products and instruments mainly include the following areas
- laser safety Laser products and instruments must comply with ANSI Z136 standard (Laser Technology Society of America’s standard for the safety of laser products and instruments). ANSI Z136 standard for the use of laser products and instruments for the safety of a comprehensive regulation, and provides for the relevant laser level and laser irradiation equipment, the use of the site, the use of personnel conditions, and so on. 2. laser power density Laser products and instruments must be certified by the FDA in order to be sold and used in the United States.
- Laser power density The power density of laser products and instruments must comply with FDA standards. According to the different levels of beam type, wavelength, power and duration, the FDA has made detailed provisions for power density standards.
- control measures The FDA requires that laser products and instruments must be equipped with relevant guidelines and safety warning signs, the user needs to follow the appropriate control measures in the use of the operation. At the same time, laser products and instruments for sale and use must provide operational training.
Ⅱ, Scope of FDA Approval for Laser Products and Instruments
The scope of FDA certification of laser products and instruments is very broad, mainly including the following areas
- medical field medical laser equipment, laser surgical equipment, laser skin treatment equipment, laser ophthalmic equipment, laser dental equipment, etc. 2. industrial field laser marking equipment
- industrial field laser marking equipment, laser cutting equipment, laser welding equipment, laser measuring equipment, etc. 3. communication field laser, laser cutting equipment, laser measuring equipment, etc.
- communication field Lasers, laser fiber amplifiers, laser transmission equipment, etc.
- Consumer goods field Laser printers, laser pointers, etc.
Laser products and instruments FDA certification has become a necessary condition to enter the U.S. market. In order to ensure product quality and safety, in the face of complex certification processes and professional certification standards, it is recommended that companies in need can look for a professional certification body to apply for certification, to ensure that the product successfully passed the FDA certification.
Ⅲ, Specialized Knowledge
- Classification of laser products and instruments
According to ANSI Z136 standard, laser products and instruments are divided into four classes 1, 2, 3R, 3B and 4, of which classes 1 and 2 are not hazardous to the human eye, class 3R and the following will be hazardous to the human eye for a short period of time, class 3B will be hazardous to a greater extent, and class 4 is the most serious. When applying for FDA approval, laser products and instruments need to specify their class.

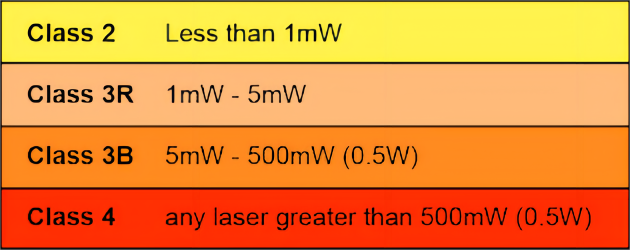
- Laser Hazards
The hazards of lasers are many and varied and include eye and skin damage, including blindness and skin cancer. Laser products and devices must be used and sold in a manner that is safe for both the operator and the consumer, and therefore need to comply with the relevant certification standards.
- Safety Control Measures
When using laser products and instruments, it is necessary to follow safety control measures, including wearing safety glasses or masks, setting safety distances, and using protective covers. At the same time, when selling laser products and instruments, it is necessary to provide instructions for use as well as warning signs for safety control measures.

Ⅳ, Questions and Answers
-
Do all laser products and instruments require FDA approval?
A All laser products and instruments sold and used in the U.S. market need to be FDA-certified. 2.
-
How long is FDA approval valid?
A The validity period of FDA approval varies from 3 to 5 years depending on the laser product or device and its classification level. 3.
-
What documents are required when applying for FDA approval?
When applying for FDA certification, it is necessary to provide detailed descriptions and lists of relevant products, as well as the required test reports, certificates and other related materials. In addition, it is necessary to provide information on the production process, quality control and other aspects.
Ⅴ, Conclusion:
If you want to use or sell laser cleaning machines, laser welding machines, laser cutting machines, laser marking machines in the U.S. or North America, you must follow the local government certification requirements, product manufacturing standards to ensure that you or your customers are safe to use the laser equipment.
All MOZLASER laser equipment including laser welding machines, laser cleaning machines, laser cutting machines have been registered with the FDA and labeled with warning labels in accordance with FDA standards, so you can purchase with confidence, if you want to distribute or represent our laser equipment in your local area, please feel free to contact us at any time.


